Objectives
The Acetyl Salicylic Elimination Trial (ASET) demonstrated the feasibility and safety of aspirin-free prasugrel monotherapy following optimal everolimus-eluting platinum chromium stent implantation by showing no occurrence of major ischemic events including stent thrombosis among selected low-risk patients with stable coronary artery disease (CAD) in Brazil1.
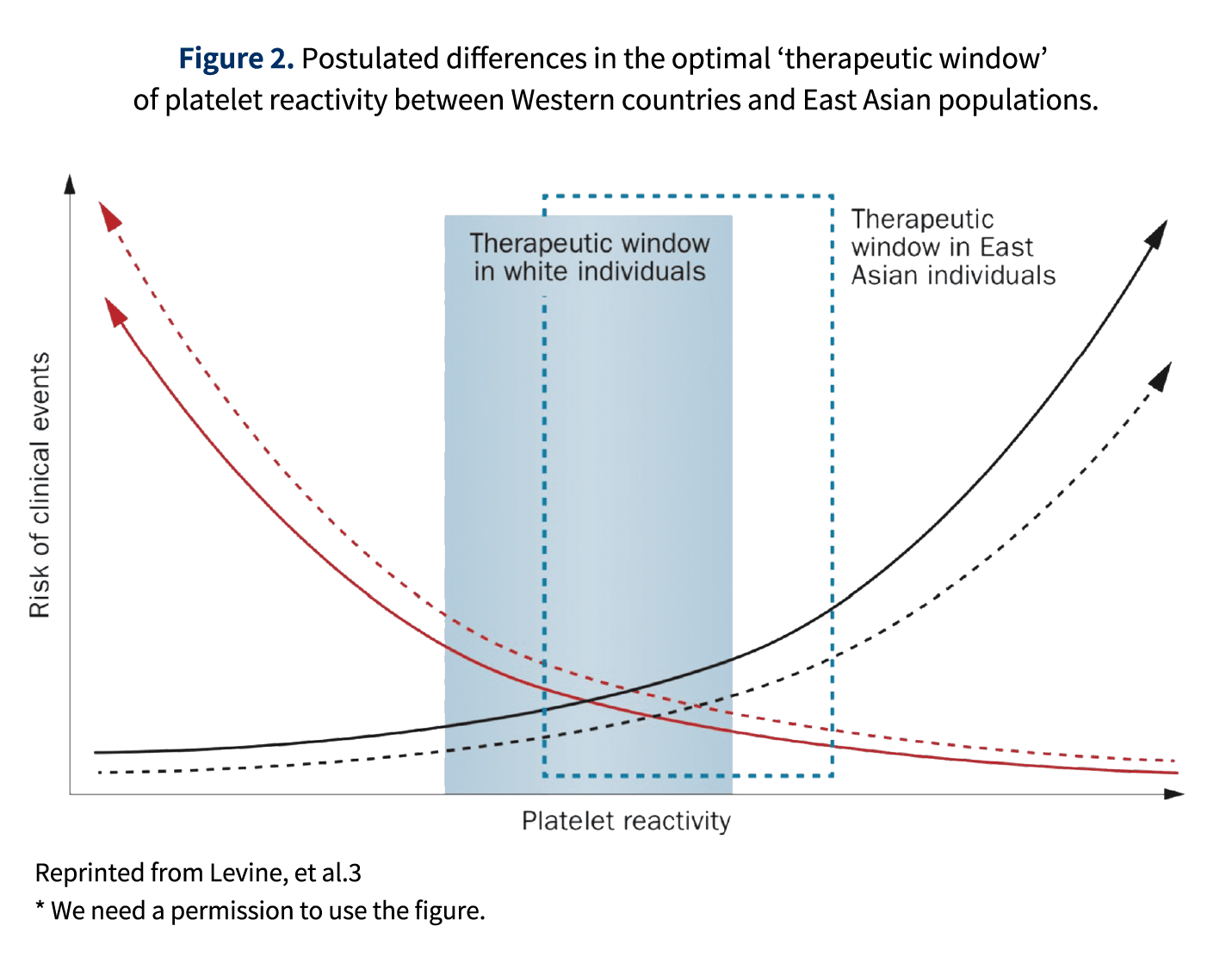
It has been elucidated that Asian population have a relatively high bleeding risk with a relatively low ischemic risk compared to Western population 2, 3. Based on the several evidences dedicated for Japanese CAD patients 4, 5, adjusted low-dose prasugrel has been implemented as a standard of care for secondary prevention after PCI in Japanese patients.
The aspirin-free strategy with adjusted low-dose prasugrel monotherapy may have a potential to avoid an unnecessary risk of bleeding without increase in ischemic events in selected Japanese patients when an optimal everolimus-eluting platinum chromium stent implantation is achieved 6.
The objective of the ASET-JAPAN study is to investigate feasibility and safety of single antiplatelet therapy with low-dose prasugrel immediately after PCI with optimal everolimus-eluting platinum chromium stent implantation in Japanese population.