Objectives
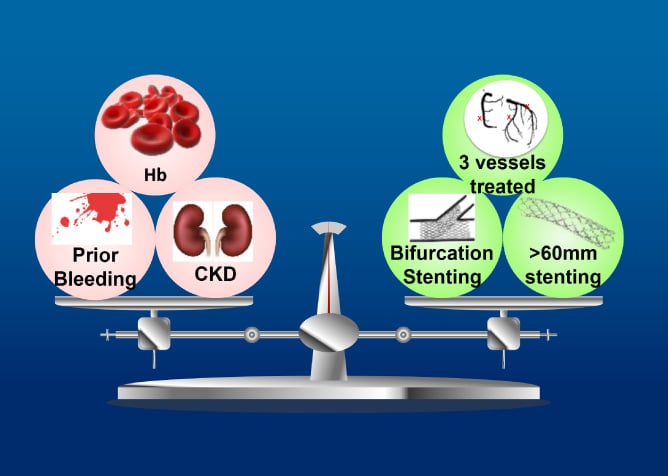
The objective of the ASET-JAPAN study is to investigate feasibility and safety of single antiplatelet therapy with low-dose prasugrel immediately after PCI with optimal everolimus-eluting platinum chromium stent implantation in Japanese population.

Study design
The ASET Japan Pilot study is a multicenter, single arm, open-label trial of single antiplatelet therapy with low dose prasugrel for patients undergoing successful.

Endpoints
For each endpoint
- Primary Ischemic Endpoint
- Primary Bleeding Endpoint
- Secondary endpoints (evaluated at each follow-up visit/contact)
News
-
2022.9.12
The target number of 200 cases for Phase1 has been achieved. -
2021.12.22
Start case registration(Phase2)
Case registration for Phase2 has started.
Participating Facilities
Principle Investigators
-

Yukio Ozaki
Fujita Health University Okazaki Medical Center
-

Kengo Tanabe
Mitsui Memorial Hospital
-

Takashi Muramatsu
Fujita Health University
-

Patrick W. Serruys
National University of Ireland Galway (NUIG)
-

Yoshinobu Onuma
National University of Ireland Galway (NUIG)
Investigators
-

Ken Kozuma
Teikyo University Hospital
-

Yoshihiro Morino
Iwate Medical University Hospital
-

Gaku Nakazawa
Kindai University Hospital
-

Takayuki Okamura
Yamaguchi University Hospital
-

Hiroki Tateishi
Yamaguchi University Hospital
-

Yosuke Miyazaki
Yamaguchi University Hospital
-

Yuki Ishibashi
St. Marianna University Hospital
-

Hideyuki Kawashima
Teikyo University Hospital
-

Taku Asano
St Luke’s international hospital
-

Shimpei Nakatani
JCHO Hoshigaoka Medical Center
-

Kuniaki Takahashi
Kindai University Hospital
-

Yuki Katagiri
Sapporo Higashi Tokushukai Hospital
-

Kogame Norihiro
Toho University Ohashi Medical Center
Patients Enrolled
Phase1
206
Phase2
101
If you are interest in the ASET study and would like to joint our study,
please contact with us through E-mail without any hesitation.