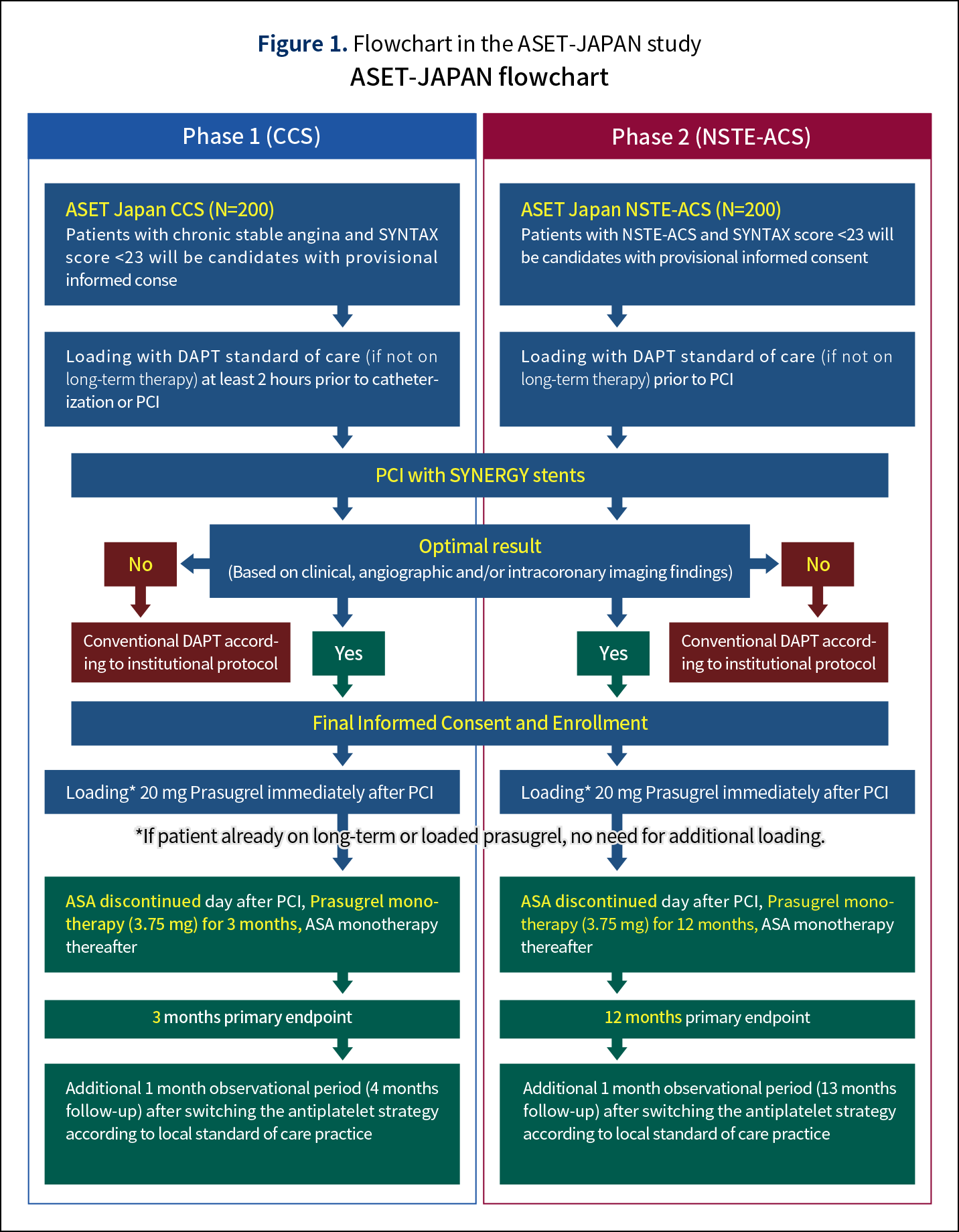
Study design
The ASET Japan Pilot study is a multicenter, single arm, open-label trial of single antiplatelet therapy with low dose prasugrel for patients undergoing successful and optimal PCI for chronic coronary syndrome (CCS: phase 1) and non-ST elevation acute coronary syndromes (NSTE-ACS: phase 2).
Standard dual antiplatelet therapy according to local practice will be performed prior to PCI. After PCI, if the results are considered to be optimal by the operator, only then patients will be enrolled in the study and loaded with 20 mg of prasugrel followed by prasugrel only (3.75 mg once a day) for 3 months for CCS patients or 12 months for NSTE-ACS patients. If prasugrel is initiated prior to PCI more than 5 days ago, loading of prasugrel will be skipped. Aspirin and other P2Y12 receptor inhibitors except for prasugrel will be discontinued just after PCI.
The “optimal stent implantation” will be determined at the discretion of the investigator, but it is typically a combination of successful stent implantation at the target lesion with absence of significant residual diameter stenosis, edge dissection, thrombus, major side branch occlusion, “no-reflow” at the end of the procedure, major stent under expansion or major stent incomplete apposition and absence of persistent chest pain after the procedure.
For safety reason, this study set a stopping rule based on the occurrence of definite stent thrombosis or sudden death. Furthermore, an independent Data Safety and Monitoring Board (DSMB) will monitor the individual and collective safety of the patients during study period.